Chapter
1
Introduction 1.1 The origin of Coal
Coal is a non-renewable energy source formed by dead plants that have been compressed and heated. It all starts with the plants that lived 300 million years ago. The plants that died in swamps were covered in layers of water and dirt, compressing and heating the plants. Through this process the energy is trapped and forms coal. Since coal is combustible, we then can burn the coal to get the trapped energy.1.1.1 BackgroundThe most favorable period for the formation of coal was 360-290 million years ago, during the carboniferous period (which means “coal-bearing”). However, lesser amounts continued to form in some parts of the earth during all subsequent periods, in particular the Permian period (290-250 million years ago), and throughout the Mesozoic era, 250-65 million years ago. The accumulating plant matter buried during the Tertiary period, i.e. less than 65 million years ago, is generally less developed-it often comes in the form of lignite, which still contains a lot of volatile matter (bitumen and wood residue) and has low carbon content. However, there is also some high quality coal from the Tertiary period, coal that matured early, heated by plate tectonics. This material was not buried deep enough to contain basic carbon. 1.2 Types of Coal
Coal is classified into four main ranks: lignite, sub-bituminous, bituminous, anthracite, depending on the amounts and types of carbon it contains and on the amount of heat energy it can produce. The rank of a deposit of coal depends on the pressure and heat acting on the plant debris as it sank deeper and deeper over millions of years. [10]1.2.1 PEATPeat is not defined as a coal, but it is an important early material formed in the coalification process. Peat is a soft organic material consisting of partly decayed plant matter together with deposited minerals. Peat forms in wetlands or peatlands, variously called bogs, moors, muskegs, pocosins, mires, and peat swamp forests. peat is the earliest stage in the formation of coal.
1.2.2 LIGNITELignite, or brown coal, is the lowest rank of coal. It has 25–35 percent fixed-carbon content, but the lowest heating value, 5,500–8,300 Btu/lb (5.8–8.8 million joules/lb) of all coals. Because of its low energy density, lignite coal is inefficient to transport and is not traded extensively on the world market compared with higher coal grades. It is often burned in power stations constructed very close to mines, so-called mine mouth power plants. It has a higher content of volatile matter and moisture.1.2.3 SUB-BITUMINOUS COALLignite coal that has been subjected to longer and deeper burial is converted to a darker and harder coal known as Sub-bituminous Coal. Sub-bituminous coal has a higher heating value than lignite. Sub-bituminous coal has 46–60 percent fixed-carbon content and a heating value of 8,300–13,000 Btu/lb (8.8–13.7 million joules/lb). Although its heat value is lower, sub-bituminous coal generally has a lower sulfur content than other types, which makes it attractive for use because it is cleaner burning.1.2.4 BITUMINOUS COALBituminous coal is a soft coal that produces smoke and ash when burned, has 46–86 percent fixed-carbon content and a heating value of 11,000–15,000 Btu/lb (11.6–15.8 million joules/lb). Bituminous coal is considered a sedimentary rock because is a product of the deep burial and compaction of plant and mineral matter. It is usually black, sometimes dark brown, often with well-defined bands of bright and dull material. Bituminous coal is the most abundant economically recoverable coal globally and the main fuel burned in steam turbine-powered electric generating plants. Some bituminous coals, known as metallurgical or coking coals, have properties that make them suitable for conversion to coke used in steel-making.1.2.5 ANTHRACITEThe highest rank of coal is anthracite, a hard black coal that burns with little flame and smoke, has the highest fixed-carbon content, 86–98 percent, and a heating value of 13,500– 15,600 Btu/lb (14.2–16.5 million joules/lb). 1.3 COAL TESTING
1.3.3.1 FUEL RATIOFuel ratio is the ratio of FIXED CARBON to VOLATILE MATTER i.e. FC : VM. According to their fuel ratios, coals have been classified. [14]TABLE 1.1 LEAST FUEL RATIO OF DIFFERENT TYPES OF COAL
1.6
Process Description
𝐶 +
𝑂2↔ 𝐶𝑂𝐶 + 𝑂2 ↔ 𝐶𝑂2 (Combustion)
· Fluidized-bed Gasifier
[8]Kentaro et al. (2011) worked on analysis of an updraft biomass gasifier with high temperature steam using a numerical model in Japan. they found that high temperature steam gasification was a gasification technology which utilizes super-heated steam at a temperature above 1273 K. Their paper addressed the performance analysis of an updraft that high temperature steam gasification gasifier using a numerical model. The experimental data was obtained from a demonstration-scale gasifier that was successfully simulated by the developed model. The calculation results showed 150–300 K temperature difference between gas phase and solid phase throughout the bed. Among a number of reactions, char gasification and water–gas shift reaction at char gasification zone played a major role to determine the syn-gas composition. Steam temperature, the ratio of steam to biomass and biomass feed rate affected the syn-gas composition while biomass particle diameter showed no significant effect. For the steam temperature and the ratio of steam to biomass, the difference of solid temperature at the bottom of gasifier determined the syn-gas composition. For biomass feed rate, the ratio of unreacted char extracted from the bottom of gasifier to supplied biomass determined the syngas composition.
3.1 Major Components:
ρ =
V=
= 0.03767
V= 61023.7 × 0.03767 = 2298.762
After the calculation for 35kg coal volume is 2298.76
V =
*h
let D = 10 in then by putting allvalues in above equation ;2298.762 = (0.785)
.h
then h = 29.283 in≈ 30 in
IN COUNTERCURRENT FIXED-BED COAL GASIFICATION Paper Submitted to the 23rd
International Symposiumof the Combustion Institute, 1990
ρ: Density
m: meter
S: Second
h: Hour
min: Minute
T: Total
kg: Kilogram
KJ: Kilo Joule
l: Liter
J: Joule
mm: Millimeter
v: Velocity
m: Mass
C0: Celsius
C: Carbon
H: Hydrogen
O: Oxygen
N: Nitrogen
S: Sulphur
I.C: Internal combustion
%: Percentage
Coal Testing may be divided into three categories 1. Proximate Analysis 2. Ultimate Analysis 3. Miscellaneous Analysis1.3.1 PROXIMATE ANALYSISProximate Analysis enables us to understand the percentage by weight of Fixed Carbon, Volatile Matter, Ash and Inherent Moisture content of a sample of coal.1.3.2 ULTIMATE ANALYSISUltimate Analysis Test is done to determine the element composition of coal which includes carbon, hydrogen, nitrogen, sulphur and oxygen.1.3.3 MISCELLANOUS ANALYSISMiscellaneous analysis is a collective category for various types of physical and chemical tests for coal that are commonly requested by coal producers and buyers. One of them are listed below:
1.4 COAL RESERVES IN PAKISTAN
The bulk of Pakistan’s indigenous coal resources lies in Sindh. The largest reserves, 175 billion tones of lignite coal, are located in the Thar Desert of Sindh. Thar coal is yet to be developed for mining and power generation. Thar Coal presents an electricity generation potential of 100,000 MW, at estimated consumption of 536 million tones/year. In addition to this, there are lignite coal reserves in Lakhra, Sonda, Indus East and other coalfields of Sindh. In Balochistan and Punjab, coal has been continuously mined since before independence. Good quality Sub-bituminous coal is available in various coalfields of Balochistan and Punjab, which coalfields are considered suitable for power generation. Some small coal reserves are also located in NWFP and AJK, and are being mined on a small scale. An estimate of coal reserves in different provinces of Pakistan is tabulated in the table 2. [11]TABLE 1.2 PROVINCE WISE COAL RESERVES
1.5 Coal Gasifier
The gasification process takes place at temperatures in the range of 700°C to 1000°C. The exact temperature depends on the characteristics of the feedstock, in particular the softening and melting temperatures of the ash. In the discussion of the theoretical background to any chemical process, it is necessary to examine both the thermodynamics (i.e., the state to which the process will move under specific conditions of pressure and temperature, given sufficient time) and the kinetics (i.e., what route will it take and how fast will it get there). over the whole temperature range described above, the reaction rates are sufficiently high that modeling on the basis of the thermodynamic equilibrium of the main gaseous components and carbon (which we will assume for the present to be graphite) gives results that are close enough to reality that they form the basis of most commercial reactor designs. This applies unconditionally for all entrained slagging gasifiers and may also be applied to most fluid bed gasifiers and even to moving-bed gasifiers. [9]1.5.1 Historical BackgroundIn the 1850s every small to medium-sized town and city had a gas plant to provide for street lighting. Subscribing customers could also have piped lines to their houses. By this era, gas lighting became accepted. Gaslight trickled down to the middle class and later came gas cookers and stoves.The 1860s were the golden age of coal gas development. Scientists like Kekulé (Germany) and Perkin (uk) cracked the secrets of organic chemistry to reveal how gas is made and its composition. In the 1850s, processes for making Producer gas and Water gas from coke were developed. Unenriched water gas (composed of carbon monoxide and hydrogen) may be described as Blue water gas (BWG).
Coal blend from storage pile is transferred in top of the gasifier through hopper. Coal moves down slowly subjected to drying, devolatilization, partial combustion and gasification. Ash from bottom of the gasifier is collected in ash pit. In the bottom of gasifier, devolatilized char is partially combusted in the presence of oxygen producing carbon dioxide and releasing heat that fulfil the endothermic gasification heat requirement. Carbon dioxide and steam react with carbon forming carbon monoxide, hydrogen and methane called heterogeneous reactions. These reactions are endothermic and their heat requirement is fulfilled by combustion zone. Sulphur in coal produce hydrogen silphide. Syngas received from the gasifier at 1300 0C and 30 bar, contain impurities like tar, soots, sulphur compounds and carbonaceous compounds. Operation for the removal of these impurities is carried out at low temperature as H2S removal is operated at low temperature, in addition to this, at high temperature solid-gas separation become difficult hence making soots and tar remain suspended in syngas. To optimize the process WHB is applied to generate steam from the raw syngas. This steam is utilized in gasifier. After WHB raw syngas is transported to cyclone separator for the removal of solid particles. For the fine removal of tar and soots high efficient cyclone separator is applied, solid particles are collected in soot pit. [9]1.6.1 COAL GASIFICATION CHEMISTRY
Driving off
water with heatIntroduction 1.1 The origin of Coal
Coal is a non-renewable energy source formed by dead plants that have been compressed and heated. It all starts with the plants that lived 300 million years ago. The plants that died in swamps were covered in layers of water and dirt, compressing and heating the plants. Through this process the energy is trapped and forms coal. Since coal is combustible, we then can burn the coal to get the trapped energy.1.1.1 BackgroundThe most favorable period for the formation of coal was 360-290 million years ago, during the carboniferous period (which means “coal-bearing”). However, lesser amounts continued to form in some parts of the earth during all subsequent periods, in particular the Permian period (290-250 million years ago), and throughout the Mesozoic era, 250-65 million years ago. The accumulating plant matter buried during the Tertiary period, i.e. less than 65 million years ago, is generally less developed-it often comes in the form of lignite, which still contains a lot of volatile matter (bitumen and wood residue) and has low carbon content. However, there is also some high quality coal from the Tertiary period, coal that matured early, heated by plate tectonics. This material was not buried deep enough to contain basic carbon. 1.2 Types of Coal
Coal is classified into four main ranks: lignite, sub-bituminous, bituminous, anthracite, depending on the amounts and types of carbon it contains and on the amount of heat energy it can produce. The rank of a deposit of coal depends on the pressure and heat acting on the plant debris as it sank deeper and deeper over millions of years. [10]1.2.1 PEATPeat is not defined as a coal, but it is an important early material formed in the coalification process. Peat is a soft organic material consisting of partly decayed plant matter together with deposited minerals. Peat forms in wetlands or peatlands, variously called bogs, moors, muskegs, pocosins, mires, and peat swamp forests. peat is the earliest stage in the formation of coal.
1.2.2 LIGNITELignite, or brown coal, is the lowest rank of coal. It has 25–35 percent fixed-carbon content, but the lowest heating value, 5,500–8,300 Btu/lb (5.8–8.8 million joules/lb) of all coals. Because of its low energy density, lignite coal is inefficient to transport and is not traded extensively on the world market compared with higher coal grades. It is often burned in power stations constructed very close to mines, so-called mine mouth power plants. It has a higher content of volatile matter and moisture.1.2.3 SUB-BITUMINOUS COALLignite coal that has been subjected to longer and deeper burial is converted to a darker and harder coal known as Sub-bituminous Coal. Sub-bituminous coal has a higher heating value than lignite. Sub-bituminous coal has 46–60 percent fixed-carbon content and a heating value of 8,300–13,000 Btu/lb (8.8–13.7 million joules/lb). Although its heat value is lower, sub-bituminous coal generally has a lower sulfur content than other types, which makes it attractive for use because it is cleaner burning.1.2.4 BITUMINOUS COALBituminous coal is a soft coal that produces smoke and ash when burned, has 46–86 percent fixed-carbon content and a heating value of 11,000–15,000 Btu/lb (11.6–15.8 million joules/lb). Bituminous coal is considered a sedimentary rock because is a product of the deep burial and compaction of plant and mineral matter. It is usually black, sometimes dark brown, often with well-defined bands of bright and dull material. Bituminous coal is the most abundant economically recoverable coal globally and the main fuel burned in steam turbine-powered electric generating plants. Some bituminous coals, known as metallurgical or coking coals, have properties that make them suitable for conversion to coke used in steel-making.1.2.5 ANTHRACITEThe highest rank of coal is anthracite, a hard black coal that burns with little flame and smoke, has the highest fixed-carbon content, 86–98 percent, and a heating value of 13,500– 15,600 Btu/lb (14.2–16.5 million joules/lb). 1.3 COAL TESTING
1.3.3.1 FUEL RATIOFuel ratio is the ratio of FIXED CARBON to VOLATILE MATTER i.e. FC : VM. According to their fuel ratios, coals have been classified. [14]TABLE 1.1 LEAST FUEL RATIO OF DIFFERENT TYPES OF COAL
RANK OF COAL
|
LEAST FUEL RATIO
|
Anthracite
|
10
|
Semi-Anthracite
|
6-10
|
Bituminous
|
3 or less
|
Semi-Bituminous
|
3-6
|
PROVINCE
|
RESERVES IN
MILLION TONNES
|
HEATING VALUE
BTU/LB
|
Sindh
|
184,623
|
5,219-13,555
|
Punjab
|
235
|
9,472-15,801
|
Balochistan
|
217
|
9,637-15,499
|
KPK
|
91
|
9,386-14,217
|
AJK
|
9
|
7,336-12,338
|
Total=185175
|
𝐶 +
· Fluidized-bed Gasifier
- Drying
- Pyrolysis
- Reduction
- Combustion
- The flow of the hot gases up from the combustion zone preheats the coal leading
- to maximum heat economy.
- High carbon conversion is assured by plug flow of solids through the gasification
- and combustion zones and the relatively long residence times of the fuel in the
- vessel.
- The product gas exits relatively cool temperatures and without contamination of
- solids.
- The feed coal has to be minimally pre-treated
- Good thermal efficiency
- Little tendency towards slag formation
- Fixed gasifiers are simple and reliable
- Fuel flexibility, can gasify a wide range of feedstock.
- Moderate oxidant and steam requirements.
- Extensive char recycling is required.
- Increase reactor size
- Fluidized bed gasifier lie in the rather high tar content of the product gas (up to 500 mg/m³ gas)
- The incomplete carbon burn-out.
- Pumping requirement
- Fuel flexibility, can accept a variety of solid feedstocks
- Can either be oxygen or air blown, but most commercial plants are oxygen blown
- Uniform temperature within the reactor.
- Slagging operation.
[8]Kentaro et al. (2011) worked on analysis of an updraft biomass gasifier with high temperature steam using a numerical model in Japan. they found that high temperature steam gasification was a gasification technology which utilizes super-heated steam at a temperature above 1273 K. Their paper addressed the performance analysis of an updraft that high temperature steam gasification gasifier using a numerical model. The experimental data was obtained from a demonstration-scale gasifier that was successfully simulated by the developed model. The calculation results showed 150–300 K temperature difference between gas phase and solid phase throughout the bed. Among a number of reactions, char gasification and water–gas shift reaction at char gasification zone played a major role to determine the syn-gas composition. Steam temperature, the ratio of steam to biomass and biomass feed rate affected the syn-gas composition while biomass particle diameter showed no significant effect. For the steam temperature and the ratio of steam to biomass, the difference of solid temperature at the bottom of gasifier determined the syn-gas composition. For biomass feed rate, the ratio of unreacted char extracted from the bottom of gasifier to supplied biomass determined the syngas composition.
3.1 Major Components:
- Gasifier Reactor
- Water jacket
- Cyclone Filter
- External body
- Grate
- Blower
- Ash collector
- Ball valve
- Iron Frame
- Outer Coupling
- Steel Nuts
- Pipes
ρ =
V=
V= 61023.7 × 0.03767 = 2298.762
After the calculation for 35kg coal volume is 2298.76
V =
let D = 10 in then by putting allvalues in above equation ;2298.762 = (0.785)
then h = 29.283 in≈ 30 in
Surface Area(
|
Steam Produced(kg)
|
2365
|
80000
|
0.324128
|
10
|
% by mass
|
|
Carbon
|
58
|
Hydrogen
|
4.16
|
Oxygen
|
11.8
|
Nitrogen
|
1.02
|
Sulfur
|
0.56
|
Ash
|
13.99
|
Combination
|
Mass (per kg)
|
O2 (required)
|
Carbon
|
0.58
|
1.546
|
Hydrogen
|
0.041
|
0.082
|
Sulfer
|
0.0056
|
0.0056
|
Oxygen
|
0.11
|
-0.11
|
Nitrogen
|
0.01
|
-
|
Ash
|
0.139
|
-
|
Combination
|
Mass (per kg)
|
Products
|
Carbon
|
0.58
|
2.156
|
Hydrogen
|
0.041
|
0.082
|
Sulfer
|
0.0056
|
0.0056
|
Oxygen
|
0.11
|
-0.11
|
Nitrogen
|
0.01
|
-
|
Ash
|
0.139
|
-
|
Insert the plate between top roll and bottom roll by adjusting the top
roll manually.
|
|
Apply pressure on the plate by lowering the top
roll, then start the main motor in forward direction till one end
of plate comes over the first bottom roll. Then stop the main motor and
reverse its direction so that other end of the
plate will come on second bottom roll.
|
|
Repeat the same procedure by applying the pressure from top roll till you get a perfect shell as shown on figure.
|
|
For removal of rolled shell reduce the pressure of the top roll, make
the shell free between top and bottom rolls, remove tilting end bearing of
top roll manually and take out the shell
|
|
Replace that bearing and the machine is ready for next operation.
|
Surface
Area(
|
Steam
Produced(kg)
|
2365
|
80000
|
0.324128
|
10
|
% by mass
|
|
Carbon
|
90
|
Hydrogen
|
3
|
Oxygen
|
2.5
|
Nitrogen
|
1
|
Sulfur
|
0.5
|
Ash
|
3
|
Combination
|
Mass (per kg)
|
O2 (required)
|
Carbon
|
0.9
|
2.4
|
Hydrogen
|
0.03
|
0.24
|
Sulfer
|
0.005
|
0.005
|
Oxygen
|
0.025
|
-0.025
|
Nitrogen
|
0.01
|
-
|
Ash
|
0.03
|
-
|
Combination
|
Mass (per kg)
|
Prodects
|
Carbon
|
0.9
|
3.3
|
Hydrogen
|
0.03
|
0.27
|
Sulfer
|
0.005
|
0.01
|
Oxygen
|
0.025
|
-
|
Nitrogen
|
0.01
|
0.01
|
Ash
|
0.03
|
-
|
% by mass
|
|
Carbon
|
58
|
Hydrogen
|
4.16
|
Oxygen
|
11.8
|
Nitrogen
|
1.02
|
Sulfur
|
0.56
|
Ash
|
13.99
|
Combination
|
Mass (per kg)
|
O2 (required)
|
Carbon
|
0.58
|
1.546
|
Hydrogen
|
0.041
|
0.082
|
Sulfer
|
0.0056
|
0.0056
|
Oxygen
|
0.11
|
-0.11
|
Nitrogen
|
0.01
|
-
|
Ash
|
0.139
|
-
|
Combination
|
Mass (per kg)
|
Products
|
Carbon
|
0.58
|
2.156
|
Hydrogen
|
0.041
|
0.082
|
Sulfer
|
0.0056
|
0.0056
|
Oxygen
|
0.11
|
-0.11
|
Nitrogen
|
0.01
|
-
|
Ash
|
0.139
|
-
|
Coal
type
|
Air
required
|
Gas
produced
|
Anthracitic
|
67.5
|
17.95
|
Indonesian
|
39.7
|
10.65
|
Sr.No
|
Coal
quantity
(kg)
|
Time
t (minutes)
|
Velocity
of Gas V (m/s)
|
Discharge
Q=A.V
(
|
Mass
flow rate m0=Q.ρ
(kg/s)
|
Mass
M=m0.t
(kg)
|
1.
|
5
|
60
|
4.5
|
0.0091125
|
0.002369
|
8.5284
|
2.
|
3
|
36
|
4.1
|
0.0083025
|
0.002369
|
5.11
|
3.
|
2
|
30
|
3.5
|
0.007087
|
0.0011842
|
3.31
|
Sr.No
|
Coal
quantity (kg)
|
Time
(minutes)
|
Mass
of Gas(kg)
Theoretical
|
Mass
of Gas(kg)
Experimental
|
1.
|
5
|
60
|
10.65
|
8.528
|
2.
|
3
|
36
|
6.39
|
5.11
|
3.
|
2
|
30
|
4.24
|
3.31
|
Sr.No
|
Theoretical
values
|
Experimental
values
|
||
Water
(kg)
|
Steam
(kg)
|
Water
(kg)
|
Steam
(kg)
|
|
1.
|
10.2
|
10
|
9
|
8.82
|
2.
|
6.15
|
6
|
5
|
4.9
|
3.
|
5.1
|
5
|
4.5
|
4
|
Sr.No
|
Components
|
Inside
temperature
(C0)
|
Outside
temperature
(C0)
|
1.
|
Cyclone
|
150
|
88
|
2.
|
Steam
pipe
|
250
|
93
|
ρ: Density
m: meter
S: Second
h: Hour
min: Minute
T: Total
kg: Kilogram
KJ: Kilo Joule
l: Liter
J: Joule
mm: Millimeter
v: Velocity
m: Mass
C0: Celsius
C: Carbon
H: Hydrogen
O: Oxygen
N: Nitrogen
S: Sulphur
I.C: Internal combustion
%: Percentage
Coal Testing may be divided into three categories 1. Proximate Analysis 2. Ultimate Analysis 3. Miscellaneous Analysis1.3.1 PROXIMATE ANALYSISProximate Analysis enables us to understand the percentage by weight of Fixed Carbon, Volatile Matter, Ash and Inherent Moisture content of a sample of coal.1.3.2 ULTIMATE ANALYSISUltimate Analysis Test is done to determine the element composition of coal which includes carbon, hydrogen, nitrogen, sulphur and oxygen.1.3.3 MISCELLANOUS ANALYSISMiscellaneous analysis is a collective category for various types of physical and chemical tests for coal that are commonly requested by coal producers and buyers. One of them are listed below:
1.4 COAL RESERVES IN PAKISTAN
The bulk of Pakistan’s indigenous coal resources lies in Sindh. The largest reserves, 175 billion tones of lignite coal, are located in the Thar Desert of Sindh. Thar coal is yet to be developed for mining and power generation. Thar Coal presents an electricity generation potential of 100,000 MW, at estimated consumption of 536 million tones/year. In addition to this, there are lignite coal reserves in Lakhra, Sonda, Indus East and other coalfields of Sindh. In Balochistan and Punjab, coal has been continuously mined since before independence. Good quality Sub-bituminous coal is available in various coalfields of Balochistan and Punjab, which coalfields are considered suitable for power generation. Some small coal reserves are also located in NWFP and AJK, and are being mined on a small scale. An estimate of coal reserves in different provinces of Pakistan is tabulated in the table 2. [11]TABLE 1.2 PROVINCE WISE COAL RESERVES
1.5 Coal Gasifier
The gasification process takes place at temperatures in the range of 700°C to 1000°C. The exact temperature depends on the characteristics of the feedstock, in particular the softening and melting temperatures of the ash. In the discussion of the theoretical background to any chemical process, it is necessary to examine both the thermodynamics (i.e., the state to which the process will move under specific conditions of pressure and temperature, given sufficient time) and the kinetics (i.e., what route will it take and how fast will it get there). over the whole temperature range described above, the reaction rates are sufficiently high that modeling on the basis of the thermodynamic equilibrium of the main gaseous components and carbon (which we will assume for the present to be graphite) gives results that are close enough to reality that they form the basis of most commercial reactor designs. This applies unconditionally for all entrained slagging gasifiers and may also be applied to most fluid bed gasifiers and even to moving-bed gasifiers. [9]1.5.1 Historical BackgroundIn the 1850s every small to medium-sized town and city had a gas plant to provide for street lighting. Subscribing customers could also have piped lines to their houses. By this era, gas lighting became accepted. Gaslight trickled down to the middle class and later came gas cookers and stoves.The 1860s were the golden age of coal gas development. Scientists like Kekulé (Germany) and Perkin (uk) cracked the secrets of organic chemistry to reveal how gas is made and its composition. In the 1850s, processes for making Producer gas and Water gas from coke were developed. Unenriched water gas (composed of carbon monoxide and hydrogen) may be described as Blue water gas (BWG).
Coal blend from storage pile is transferred in top of the gasifier through hopper. Coal moves down slowly subjected to drying, devolatilization, partial combustion and gasification. Ash from bottom of the gasifier is collected in ash pit. In the bottom of gasifier, devolatilized char is partially combusted in the presence of oxygen producing carbon dioxide and releasing heat that fulfil the endothermic gasification heat requirement. Carbon dioxide and steam react with carbon forming carbon monoxide, hydrogen and methane called heterogeneous reactions. These reactions are endothermic and their heat requirement is fulfilled by combustion zone. Sulphur in coal produce hydrogen silphide. Syngas received from the gasifier at 1300 0C and 30 bar, contain impurities like tar, soots, sulphur compounds and carbonaceous compounds. Operation for the removal of these impurities is carried out at low temperature as H2S removal is operated at low temperature, in addition to this, at high temperature solid-gas separation become difficult hence making soots and tar remain suspended in syngas. To optimize the process WHB is applied to generate steam from the raw syngas. This steam is utilized in gasifier. After WHB raw syngas is transported to cyclone separator for the removal of solid particles. For the fine removal of tar and soots high efficient cyclone separator is applied, solid particles are collected in soot pit. [9]1.6.1 COAL GASIFICATION CHEMISTRY
Heating without air
Converting coal to flammable gas
Add air to burn coalFigure 1.1: Fixed-bed Gasifier1.7.1.1 Advantages
1.7.2 Fluidized bed gasifierAir is blown through a bed of solid particles at a sufficient velocity to keep these in a state of suspension. The bed is originally externally heated and the feedstock is introduced as soon as a sufficiently high temperature is reached. The fuel particles are introduced at the bottom of the reactor, very quickly mixed with the bed material and almost instantaneously heated up to the bed temperature. As a result of this treatment the fuel is pyrolysed very fast, resulting in a component mix with a relatively large amount of gaseous materials. Further gasification and tar-conversion reactions occur in the gas phase. Most systems are equipped with an internal cyclone in order to minimize char blow-out as much as possible. Ash particles are also carried over the top of the reactor and have to be removed from the gas stream if the gas is used in engine applications.Figure 1.2: Fluidized bed gasifier1.7.2.1 ADVANTAGES
1.7.2.2 DISADVANTAGE
1.7.3 Entrained-flow gasifiersEntrained-flow reactors operate in co-current flow. Pulverized coal is entrained in the oxidant (oxygen and steam) and introduced into the gasifier (sometimes using coal water slurry [CWS] and pumped into the gasifier). The reaction takes place at a high temperature (1500 – 1900 ° C) and generates CO, H2, CO2, and other gases. Ash is removed as molten slag from the bottom of the reactor. Due to the short residence time in the reactor, high temperatures are required to ensure a good coal conversion, and therefore the operating temperature is high. The oxygen consumption is also high in the entrained-flow gasifier to maintain a high operating temperature. Due to the high operating temperature, entrained-flow gasifiers do not have any specific technical limitations on the type of feedstock used. However, coals with a high moisture or ash content require higher oxygen consumption and it reduces the heat efficiency compared with alternate processes. Figure 1.3: Entrained-flow gasifiers
1.8 Applications of GasificationCoal gasification has a wide range of applications as shown schematically in the figure below. [10]Figure 1.4: Application of Gasification
Literature Review 2.1 Literaure Review:
[1] The model used by Jayah et al., (2003) consisted of two sub models, namely Milligan’s (1994) flaming pyrolysis zone model and Chen’s (1987) gasification zone model. The flaming pyrolysis zone sub-model was used to determine the maximum temperature and the product concentration of gas leaving that zone. The gasification zone sub-model assumed that a single char particle moved vertically downwards the gasifier.
[7] Verhoeven et al. (2008) worked on Analysis and operation for optimization of an updraft gasifier unit. They found that biomass gasification requires an air flow of between 1.25 m3 and 2 m3 per kg of wood. This corresponds to equivalence ratios (the ratio of the actual fuel/air ratio to the stoichiometric fuel/air ratio.) between 0.25 and 0.4. Above this equivalence ratio, combustion takes place instead of gasification. The gas produced from the reactor has a relatively high content of tar and moisture when compared with a downdraft unit because the products of pyrolysis and drying are added to those of reduction as they flow upwards toward the reactor outlet. They recommended for more efforts should be given for better results.
Chapter 3
DESIGN GASIFIER
We have included following major components in our design:
Other components:
3.1.1 Metal Frame: It provides the compactness and portability to whole structure .And it is the External body.3.2 Calculation:3.2.1 Formulas:For discharge measurement Q = V.AFor cylinder area A =Volume of cylinder = area × height V = A × h
Density ρ =
For Volume V : Density of Coal = 929Mass = 20 kg As we know thatDensity =
1
Figure 3.1 Gasifier reactor
3.4 Water jacket
Diameter of water jacket =1.5 inches
Height of water jacket =16 inches
Thickness of water jacket = 3.5 mm
Diameter of gasifier = 10 inches
The steam is generated on the surface of the gasifier because of the heat transfer from the gasifier reactor to the water jacket.
Surface area of the gasifier =π d L
S.A =3.14×10×16=502.4
Table 3.1 Steam generated
The steam produced from water jacket is 10 kg.
Figure 3.2 Water jacket
3.5 Cyclone:
R= 3 inches
r = .75 inches
h = 10 inches
V1=
V1=
V1=169.59
V2 =
V2=
V2=123.6375
VT= v1+v2
VT= 169.59+123.6375
VT=293.1975
Figure 3.3 Cyclone
3.6 External body:
Height=30 in
Width=19 in
Thickness=1.5 in
Figure 3.4 External body
3.7 Grate:
Diameter of grate = 10 in
Coal grain size = 25-35mm
Grate hole size = 8mm
Figure 3.5 Grate
3.8 Combustion Calculation for a Coal
A coal has the following ultimate analysis:
Table 3.1 Combustion Calculation for a Coal
3.8.1 Calculate:
The volumetric air supply rate required if 5 kg/h of coal is to be burned at 20% excess air
Lay out the calculation on a tabular basis using 1 kg coal:
Table 3.2 Calculate on a tabular basis using 1 kg coal
Oxygen required to burn 1 kg coal = 1.546+0.082+0.0056-0.11=1.5236
Air required =
Assuming a density for air of 1.2 kg/m3,
Actual air supplied = 6.624 × 1.2 = 7.949 kg
For 5kg
Table 3.3 Syn gas produced
Syn gas produced from 1 kg coal = 2.156 + 0.82 + 0.0056 - 0.011 = 2.13 kg.
Syn gas produced from 5 kg of coal =10.65 kg /h.
3.9 Blower
Discharge is given by
Q = V.A
Q = A.V and also A =
For D =1 inch, A= 0.785
and V = 385
Capacity of blower required = Q = 302.2
CHAPTER 4
FABRICATION OF GASIFICATION PLANT
4.1 Fabrication:
Metal fabrication is the building of metal structures by cutting, bending, welding and assembling processes. It is a value added process that involves the construction of machines and structures from various raw materials.
v Different machines use in manufacturing of gasification plant:
Ø Shear cutting machine.
Ø Rolling machine.
Ø Grinding machine.
Ø Power hacksaw.
Ø Universal drilling machine.
Ø Welding plant.
4.1.1 Shear Cutting Machine Process:
Sheet available in market are in different sizes as compare with our required size so shear cutting machine used for cutting sheet or plates for required shapes. A punch (moving blade) is used to push a workpiece against the die or fixed blade, which is fixed. Usually, measured the point where the cutting action takes place and perpendicular to the direction of blade movement.
4.1.1.1 Shear Cutting Machine:
The basic shear frame consists of table assembly which is welded or bolted to side frames, a moving ram assembly which is powered hydraulically or mechanically and a hold down ram also fixed to the side frames. Shearing is a simple process whereby a sheet of metal is cut into smaller pieces by two knives which are positioned at an angle relative to each other. The lower knife is firmly attached into a pocket in the stationary table, while the upper blade is fixed to the moving ram assembly. The two blades are separated only by a distance measured in thousandths of an inch at the point of cut.
Used for cutting sheet for following parts
Ø Gasifier reactor
Ø Water jacket
Ø Cyclone filter
Ø External casing
Fig 4.1 Shear Cutting Machine
4.1.2 Universal Drilling Machine:
Universal drilling machine is use for hole in sheet.
Drilling is the operation of producing circular hole in work–piece by using a rotating cutter called DRILL. The machine used for drilling is called drilling machine. The most common drill used is the twist drill.
Used for making holes in flange.
Fig 4.2 Universal Drilling Machine
4.1.3 Angle Grinding Machine:
An angle grinder, also known as a side grinder or disc grinder, is a handheld power tool used for cutting, grinding. Angle grinders can be powered by an electric motor. The motor drives a geared head at a right-angle on which is mounted an abrasive disc or a thinner cut-off disc, either of which can be replaced when worn.
Used for cutting pipes and frame material.
Fig 4.3 Angle Grinding Machine
4.1.4 Hand Grinding Machine:
Grinding is a metal cutting operations performed by means of abrasive particles rigidly mounted on rotation wheel. Each of the abrasive particles act as a single point cutting tool and grinding wheel acts as a multipoint cutting tool. The grinding operation is used to finish the work pieces with extremely highly quality of surface finish and accuracy of shape and dimension. Grinding is one of the widely accepted finishing operations because it removes material in very small size of chips 0.25 to 0.50mm. It provides accuracy of the order of 0.000025mm.
A cutting disk mixture is mostly made of aluminum oxide Al2O3, silicon carbide (SiC), and other special minerals.
Diamond cutting discs is used for cutting ceramic, steel, stainless steel, in which diamond is used as coating on mixture.
Fig 4.4 Hand Grinding Machine
4.1.5 Plate Rolling Machine:
A Plate Rolling Machine is a machine that will roll different kind of metal sheet into a round or conical shape. It can be also called “Roll bending machine” “plate bending Machine” or “rolling machine”
4.1.5.1 Roll Variable Machine:
Geometry works by having all three rolls being able to move and tilt. The Top-Roll moves on the vertical plane. The Side-Rolls move on the horizontal plane. When rolling, the Top-Roll presses the metal plate between the two Side-Rolls. The advantage of having the Variable 3 Roll is the ability to roll many thicknesses and diameters of cylinders.
Fig 4.5 Roll Variable Machine
4.1.6 Welding Process:
Fusion welding
In this type welding we add filler metal to the joint
4.1.6.1 Electric Arc Welding (AW):
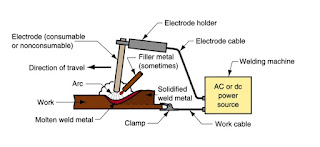
A fusion welding process in which coalescence of the metals is achieved by the heat from an electric arc between an electrode and the work. Electric energy from the arc produces temperatures ~ 10,000 F (5500 C), hot enough to melt any metal. Most AW processes add filler metal to increase volume and strength of weld joint. An electric arc = discharge of electric current across a gap in a circuit .It is sustained by an ionized column of gas (plasma) through which current flows .To initiate the arc in AW, electrode is brought into contact with work and then quickly separated from it by a short distance. A pool of molten metal is formed near electrode tip. As electrode is moved along joint, molten weld pool solidifies.
Used for joining for following parts
Ø Gasifier reactor
Ø Water jacket
Ø Cyclone filter
Ø External casing
Fig 4.6 Electric Arc Welding
Fig 4.6.1 Electric Arc Welding
4.2 Material:
We are using mild stell sheet for fabrication of different parts of prototype plant.
Main properties are given
Ø Mild Steel
Ø Melting temperature 1500c
Ø Density 7870
Ø Ultimate Tensile Strength 440 Mpa
Ø Yield Strength 370 Mpa
Ø Modulus of Elasticity205 Gpa
Ø Poission Ratio 0.29
4.3 Tool and Instrument Used:
Ø Vernier caliper
Ø Measuring tape
Ø Hammer
4.4 Components :
Ø Gasifier Reactor
Ø Water jacket
Ø Cyclone Filter
Ø Grate
Ø External body
Ø Blower
Ø Ash collector
Ø Ball valve
Ø Iron Frame
Ø Outer Coupling
Ø Steel Nuts
Ø Pipes
4.4.1 Gasifier Reactor:
It is the main part of the gasifier in which the gasification take place. It has the four zones. Combustion zone, Gasification zone, pyrolysis zone and drying zone. The gasifier reactor which is usually a cylindrical reactor and forms a packed bed on the grate. The cylinder is 30in height and 10in diameter and made up of 4mm thickness Ms Sheet. The cylinders are fixed with the flange at the top.
Fig 4.7 Gasifier Reactor
4.4.2 Water jacket:
It is used to generate the steam by utilizing waste heat of the reactor. It is made of the Ms Sheet. Its diameter is 13 inches and thickness of sheet is 3.5mm.
Fig 4.8 Water jacket
4.4.3 Cyclone Filter:
Product gases have a number of by products like tar and other solid particles which must be removed before use of coal gas. Cyclone filter is used for the removal of these by product. It is made of Ms (Mild-steel) .The taper portion of the cylinder, where tar and moisture set down that finally drain out through controlling nut. Its total height is 16 inches and diameter is 6 inches with sheet thickness of 3mm.
Fig 4.9 Cyclone Filter
4.4.4 Grate:
Grate is placed at the bottom of the gasifier. Virtually the fuel materials set burning on the grate and form a packed bed over it. The fuel ash after combustion falls through the grate and retain in the ash collector. Plate made up of 4 mm Thickness . And Grate is comprised of plate of 8mm × 8 mm hole size placed at the bottom of the gasifier.
Fig 4.10 Grate
4.4.5 Blower:
A centrifugal fan is a mechanical device for moving air or other gases increase the speed of an air stream with the rotating impellers. The gasification agent is injected from above the grate and passes through the fuel bed result hot product gases exit the top of the gasifier.
Ø V = 385
Ø Capacity of blower required = Q = 302.2
4.4.6 Ash Collector:
The fuel ash from the reactor falls through the grate keep stacking in the ash collector. An ash outlet port is used to dispose the ash from the collector. Its radius is 19 inches.
Fig 4.12 Ash Collector
4.4.7 Ball Valve:
A ball valve is a form of quarter-turn valve which uses a hollow, perforated and pivoting ball to control flow through it. It is open when the ball's hole is in line with the flow and closed when it is pivoted 90-degrees by the valve handle. The handle lies flat in alignment with the flow when open, and is perpendicular to it when closed. we are using the ball valve for one inch.
Fig 4.13 Ball Valve
4.4.8 Pipes:
A hollow cylinder or tube, used for the flow of steam and air. we are using stander size of 1inch in our project.
Fig 4.14 Pipe
4.4.9 External Body:
It is used to cover the water jacket and gasifier, the main purpose of it is to prevent heat transfer. The height of the external body is 30inches and it,s diameter is 19 inches. the thickness of external body is 1.25mm.
Fig 4.15 External Body
4.5 Assembly:
We assemble all parts together by wilding .also use flange for connecting gasifier to the water jacket.
Ø Assembly of gasifier, water jacket and external body.
Fig 4.16 Gasifier, water jacket and external body
Ø Cyclone
Fig 4.17 Cyclone
Ø Complete Assembly of Coal gasifiers
Fig 4.18 complete Assembly
Ø Actual assembly:
Figure 4.19 Actual assembly
CHAPTER 5
Experimentation and Results Analysis
5.1 Mathematical Calculation
5.1.1 Mass of Coal:
For Volume V :
Density of Coal = 929
Mass = 15 kg
As we know that
Density =
ρ =
V =
V=
1
V= 61023.7 × 0.0161= 985.31
Volume required for 15kg coal is 985.31
volume of cylinder = area × height
V = A × h
Area of cylinder =
V =
let D = 10 in then by putting all
Values in above equation ;
V = (0.785)
then
V=1256
Volume of rectifier 1256
5.2 Steam produced:
Surface area =π d L
S.A =3.14×10×16=502.4
Table 5.1 Steam produced
The steam produced from water jacket is 10 kg.
5.3 Combustion Calculation for a Coal:
5.3.1 Anthracitic coal:
A coal has the following ultimate analysis:
Table 5.2 Anthracitic coal
5.3.2 Calculate:
The volumetric air supply rate required if 5 kg/h of coal is to be burned at 20% excess air.
Lay out the calculation on a tabular basis using 1 kg coal:
Table 5.3 the calculation on a tabular basis using 1 kg coal
Oxygen required to burn 1 kg coal = 2.4 + 0.24 + 0.005 - 0.025 = 2.62 kg.
Air required =
Assuming a density for air of 1.2 kg/m3,
Actual air supplied = 11.25 × 1.2 = 13.5 kg
Table 5.4 Syn gas produced from 1 kg coal
Syn gas produced from 1 kg coal = 3.3 + 0.27 + 0.01 +0.01 = 3.59kg.
Syn gas produced from 5 kg of coal= 17.95kg/h
5.3.3 Indonesian Coal:
A coal has the following ultimate analysis:
Table 5.5 Indonesian Coal:
5.3.4 Calculate:
The volumetric air supply rate required if 5 kg/h of coal is to be burned at 20% excess air
Lay out the calculation on a tabular basis using 1 kg coal:
Table 5.6 calculation of 1 kg coal of Indonesian Coal
Oxygen required to burn 1 kg coal = 1.546+0.082+0.0056-0.11=1.5236
Air required =
Assuming a density for air of 1.2 kg/m3,
Actual air supplied = 6.624 × 1.2 = 7.949 kg
For 5kg
Table 5.7 Syn gas produced from 1 kg coal of Indonesian Coal
Syn gas produced from 1 kg coal = 2.156 + 0.82 + 0.0056 - 0.011 = 2.13 kg.
Syn gas produced from 5 kg of coal =10.65 kg /h.
5.3.4 Comparison between anthracitic and Indonesian coal :
Table 5.8 Comparison between anthracitic and Indonesian coal :
5.4 Experimental Results:
Table 5.9 Experimental Results:
5.5 Comparison between actual and mathematical readings:
Table 5.10 Comparison between actual and mathematical readings:
Graph 5.1 Graph between actual and mathematical readings
5.6 Steam production comparison between actual and theoretical:
Table5.11 Steam production comparison between actual and theoretical:
5.7 Temperature:
Table 5.12 Temperature:
Temperature of burning coal = 900 C0 -1000 C0
Temperature of reduction zone =548 C0
Temperature of drying zone = 131C0
External body temperature = 69 C0
CHAPTER 06
SAFETY AND HAZARDS
Any organization cannot survive without any safety consideration. It is very important for
an organization to safeguard the health and welfare of its employees and the general public. Safety should be in good practice; the good management practices needed to ensure the safe operations in the organization that has an impact of efficient operations. The term loss prevention refers having loss in financial caused by an accident. This loss is not only considered by replacing the cost of damaged plant and third party claims, but also the loss of earnings from lost production and lost sales opportunity.
All manufacturing processes are to some extent hazardous, but in chemical processes there are no. of hazards associated with the chemicals, equipment’s and operations. The designer must be aware of these hazards, and ensure, through the application of sound engineering practice, that the risks are reduced to acceptable levels.
6.1 SOURCES OF IGNITION:
Precautions must be taken in order to eliminate sources of ignition on chemical plant. It is
best way to work on the principle of flammable material that can be ignite of a single
leakage.
6.1.1 DUST EXPLOSIONS:
Finely divided combustible solids, if intimately mixed with air, can explode. Several
disastrous explosions have occurred in grain silos. Particular care must be taken in the
design of dryers, conveyors, cyclones, and storage hoppers for polymers and other
combustible products or intermediates.
6.1.2 ELECTRICAL EQUIPMENT:
The sparking of electrical equipment, such as motors, is a major potential source of
ignition, and flame proof equipment is normally specified. Electrically operated
instruments, controllers and computer systems are also potential sources of ignition of
flammable mixtures.
6.1.3 PRESSURE:
Over-pressure, a pressure exceeding the system design pressure, is one of the most serious hazards in chemical plant operation. Failure of a vessel, or the associated piping, can precipitate a sequence of events that culminate in a disaster. Pressure vessels are invariably fitted with some form of pressure-relief device, set at the design pressure, so that potential over-pressure is relieved in a controlled manner.
Three basically different types of relief device are commonly used:
6.1.3.1 Directly actuated valves:
Weight or spring-loaded valves that open at a predetermined pressure and which normally close after the pressure has been relieved. The system pressure provides the motive power to operate the valve.
6.1.3.2 Indirectly actuated valves:
Pneumatically or electrically operated valves, which are activated by pressure-sensing
instruments.
6.1.3.3 Bursting discs:
Thin discs of material that are designed and manufactured to fail at a predetermined
pressure, giving a full bore opening for flow.
Relief valves are normally used to regulate minor excursions of pressure; and bursting
discs as safety devices to relieve major over-pressure. Bursting discs are often used in
conjunction with relief valves to protect the valve from corrosive process fluids during
normal operation.
6.1.4 VENT PIPING:
When designing relief venting systems it is important to ensure that flammable or toxic
gases are vented to a safe location. This will normally mean venting at a sufficient height
to ensure that the gases are dispersed without creating a hazard.
6.1.5 TEMPERATURE DEVIATIONS:
Temperature deviations can cause a major accident in any plant operation. Excessive high
temperature can cause structural failure and initiate disaster. High temperature can cause
by loss of control of reactors, heaters and externally open fire.
The protection can be taken to avoid high temperature by:
1. Provision of high-temperature alarms and interlocks to shut down reactor feeds, or
heating systems, if the temperature exceeds critical limits.
2. Provision of emergency cooling systems for reactors, where heat continues to be
generated after shut-down; for instance, in some polymerization systems.
3. Structural design of equipment to withstand the worst possible temperature excursion.
4. The selection of intrinsically safe heating systems for hazardous materials.
Chapter 7
ENVIRONMENTAL IMPACT
Coal gasification is a well-proven technology that started with the production of coal gas
for urban areas, progressed to the production of fuels, such as oil and synthetic natural gas (SNG), chemicals, and most recently, to large-scale Integrated Gasification Combined Cycle (IGCC) power generation. IGCC is an innovative electric power generation concept that combines modern coal gasification technology with both gas turbine (Brayton cycle) and steam turbine (Rankine cycle) power generation. The technology is highly flexible and can be used for new applications, as well as for repowering older coal- fired plants, significantly improving their environmental performance. IGCC provides feedstock and product flexibility, greater than 40 percent thermal efficiency, and very low pollutant emissions. IGCC plants have achieved the lowest levels of criteria pollutant air emissions (NOx, SOx, CO, PM10) of any coal- fueled power plants in the world. Emissions of trace hazardous air pollutants are extremely low, comparable with those from direct-fired combustion plants that use advanced emission control technologies. Discharge of solid byproducts and wastewater is reduced by roughly 50% versus other coal-based plants, and the by-products generated (e.g., slag and sulfur) are environmentally benign and can potentially be sold as valuable products. Another significant environmental benefit is the reduction of carbon dioxide (CO2) emissions, by at least 10% per equivalent net production of electricity, due to a higher operating efficiency compared to conventional pulverized coal- fired power plants.
7.1 CRITERIA AIR POLLUTANTS:
The EPA-designated criteria air pollutants produced by the conversion of coal and other
solid carbonaceous fuels (e.g., petroleum coke) in gasification-based power cycles are
SO2, NOx, particulates, CO. The environmental benefits of the gasification steam from the capability to achieve extremely low SOX,NOX and particulate emissions from burning coal-derived gases sulfur in coal, for example is converted to hydrogen sulfide and can be captured by processes present use in chemical industry.
7.1.1 SO2 Emissions:
Pre-combustion pollution controls used in gasification have some inherent clean-up advantages. The conditions used to gasify coal – high pressure and partial oxidation- mean that the pollutants are concentrated and therefore easier to remove. sulfur remove rates are 95 to 99% or even 99.99% in most gasification applications. During high-temperature, entrained flow gasification of coal, most of the sulfur in the coal
matrix is released and converted to hydrogen sulfide (H2S), as well as a small
amount of carbonyl sulfide (COS), due to the reduced oxygen environment. These H2S,
COS and particulate contaminants are mostly removed from the syngas prior to
combustion or other forms of fuel conversion (e.g., fuel cell). Acid gas removal equipment extracts 95-99% of the H2S and COS from the fuel gas and converts it to a salable sulfur or sulfuric acid (H2SO4) byproduct. The small amount of residual sulfur that remains in the syngas is converted to SO2 in the combustion turbine and released to the atmosphere in the primary stack gas or in the secondary stack gas from the sulfur recovery equipment.[17]
7.1.2 NOx Emission
The term ―NOx‖ refers to the sum of the nitric oxide (NO) and nitrogen dioxide (NO2)
emissions from a combustion source. While most of the NOx produced during the
combustion of syngas is in the form of NO, it is subsequently oxidized to NO2 in the
atmosphere. NOx is formed in fossil combustion systems by two primary mechanisms;
―fuel NO‖ is formed via the oxidation of chemically-bound nitrogen in the fuel, and
―thermal NO‖ is formed via the dissociation of molecular nitrogen and oxygen to their
atomic forms (at high temperatures) and subsequent recombination into oxides of nitrogen. Unlike natural gas, coal contains chemically-bound nitrogen that forms most of the NOx emissions when it is fired in a typical excess-oxygen environment, such as a utility boiler. Fuel NO typically contributes over 80% of the total NOx emissions in a coal- fired combustion unit, and its formation is highly insensitive to the flame temperature.
7.1.3 CO Emissions:
CO emissions are typically the result of incomplete combustion, but can also result from
fugitive emissions.
7.1.4 Hazardous Air Pollutants:
Potential trace substance emissions from coal- fueled power plants include ionic species,
trace elements, and trace organic compounds. These trace substances can be emitted in
flue gas, aqueous discharges, and solid effluents. Ionic species of environmental concern
in the effluent streams of coal-fueled power plants include sulfate, nitrogen-containing
ions (e.g., nitrate, ammonium), chloride, fluoride, phosphate and cyanide. The ionic forms of these species in stack gases are present only in the aerosol phase. Chloride and fluoride, however, can exist as acids and, thus, may appear in the gas phase as well. Stack emissions of all ionic species are reduced to very low levels via particulate and acid gas control equipment.
7.1.5 Greenhouse Gases
The two biggest contributors to CO2 emissions are the electric power and transport sectors. Given the millions of small moving emission sources involved in transport, any significant reductions in CO2 emissions is only likely to emerge through the change to a less carbon-intensive fuel such as natural gas or hydrogen. Although the use of natural gas (at least while it lasts) would still result in large, even if lower, emissions from millions of individual sources, the use of hydrogen offers the possibility of bundling CO2 emissions at fixed locations in a manner that would allow fixation or sequestration In such a scenario there are some natural advantages to gasification over combustion technologies. CO2 capture and usage is already standard practice in almost all gasification-based ammonia plants, where the CO2 is recovered from the synthesis gas and used to manufacture urea. [18]
7.2 GASIFICATION PROVIDES SIGNIFICANT ENVIRONMENTAL
BENEFITS
1. Gasification plants produce significantly lower quantities of criteria air pollutants.
2. Gasification can reduce the environmental impact of waste disposal because it can
use waste products as feed stocks—generating valuable products from materials
that would otherwise be disposed as wastes.
3. Gasification's byproducts are non-hazardous and are readily marketable.
4. Gasification plants use significantly less water than traditional coal-based power
generation, and can be designed so they recycle their process water, discharging
none into the surrounding environment.
5. Carbon dioxide (CO2) can be captured from an industrial gasification plant using
commercially proven technologies. In fact, since 2000, the Great Plains Substitute
Natural Gas plant in North Dakota has been capturing the same amount of CO2 as
a 400 MW coal power plant would produce and sending that CO2 via pipeline to
Canada for Enhanced Oil Recovery. [13]
CHAPTER 8
CONCLUSION AND RECOMMENDATIONS
8.1 CONCLUSION
There is a huge potential for coal gasification worldwide, as the technology enables production of fuels and feedstock for many application such as transport, chemicals production, heat and power generation. As coal is the most abundant fossil resource available on earth and even low-grade coal can be used for gasification, the technology is of primary interest in many regions. Increasing gas prices and limited availability of natural gas in regional consumer markets are driving factors for investments in coal gasification technology. As a result of depleting of oil resources, there is increasing interest in using coal sources. Coal gasification is seen as an important technology component for expanding the use of coal. In Pakistan, about more than 185.5 billion tons of coal is deposited, if half of these resources are exploited properly it would be sufficient for generating 100,000 MW of electricity for 30 years. The gasifier is made up with locally available materials. The produced gas shows sustainable burning character detected by firing.
8.2 Recommendations for Future work
Based on the present study the following recommendations are made for future study
1. The produced gas should be used for running a IC engine.
2. The gasifier should be tested for other types of coal.
3. Calorific value of gas should be quantified.
4. Combustible gas like H2, CH4 should be measured.
References
[1] Jayah, N. Jense, J., Carlsenb H., and Henriksenb ,From Updraft Gasifier, Adk-Teknik Energy & Environment, Denmark Volume 25, Issue 4, October 2003.
[2] HEEYOUNG YOON, JAMES Wei and MORTON M. DENN A Model For Moving-Bed Coal Gasification Reactors AlChE Journal (Vol. 24, No. 5) (1980)
[3] Stefano, M., Michele, V., and Giorgio, C., (2011). Two-Dimensional CFD Model of an AirBlown Coal Updraft Gasifier. Fuel, In press, corrected proof, Available online 17 september, 2011.
[4] M. L Hobbs, P. T. Radulovic and L. D. Smoot CHEMICAL AND PHYSICAL PROCESSES
IN COUNTERCURRENT FIXED-BED COAL GASIFICATION Paper Submitted to the 23rd International Symposiumof the Combustion Institute, 1990
IN COUNTERCURRENT FIXED-BED COAL GASIFICATION Paper Submitted to the 23rd International Symposiumof the Combustion Institute, 1990
[5]Alejandro, G., Emilia B., and Rolando Z., Fixed (Slow-Moving) Bed Updraft Gasification of Agricultural Residues. Dept. of Chemical Engineering and Technology / Divison of Chemical Technology, Royal Institute of Technology (KTH), S-100 44 (2004) Stockholm, Sweden.
[6],Verhoeven, dr. ir. J. A. van Oijen, prof. dr. L.P.H. de Goey, Eindhoven University of Technology "DESIGN AND DEVELOPMENT OF UPDRAFT GASIFIER” (2003)
[7] Kentaro Umeki,Tomoaki Namioka, Kunio Yoshikawa"Analysis of an updraft biomass gasifier with high temperature steam using a numerical model” Volume 90, Issue 1, February 2012
[8]Michel Bellais, K.O. Davidssonb, T. Liliedahla "Pyrolysis of large coal particles: a study of shrinkageimportance in simulations” Fuel 82 (2003) 1541–1548
Books:
[9]Gasification Technologies A Primer for Engineers and Scientists John Rezaiyan Nicholas P. Cheremisinoff Published in 2005 by CRC Press.
[10]Coal gasification and its applications david a bell brian f towler maohong fan first edition 2011.
[11]Biomass Gasifcation and Pyrolysis Practical Design and Theory Prabir Basu 2010 Prabir Basu. Published by Elsevier Inc.
Web Link:
[12] https://en.wikipedia.org/wiki/Coal_gasification
[13] http://www.scirp.org/journal/PaperInformation.aspx?PaperID=37195
[14] https://www.euronuclear.org/info/encyclopedia/coalequivalent.htm
[15] https://www.researchgate.net/publication/287044440_Design_calculation_of_a
coal_gasifier_reactor
[16] https://scihub.org/
[17] http://www.ajdesigner.com/phpcyclone/cyclone_equation_radial_velocity.php
[18] https://www.undeerc.org/Equipment/Gasification-and-Gas-Cleanup-Systems/Fixed-Bed-Gasifier.aspx
Abbreviations
H: Height
A: Area
mo: Mass flow rate
S.A: Surface Area
r: Lower radius
R: Upper radius
d: Diameter
L: Length
m: meter
S: Second
h: Hour
min: Minute
T: Total
m: meter
S: Second
h: Hour
min: Minute
T: Total
t: Time
kg: Kilogram
KJ: Kilo Joule
l: Liter
J: Joule
mm: Millimeter
v: Velocity
kg: Kilogram
KJ: Kilo Joule
l: Liter
J: Joule
mm: Millimeter
v: Velocity
Q: Discharge
m: Mass
C0: Celsius
C: Carbon
H: Hydrogen
O: Oxygen
N: Nitrogen
S: Sulphur
m: Mass
C0: Celsius
C: Carbon
H: Hydrogen
O: Oxygen
N: Nitrogen
S: Sulphur
CH4: Methane
H2O: Water
H2S: Hydrogen sulphite
V: Volume
I.C: Internal combustion
I.C: Internal combustion
In: Inch
%: Percentage
%: Percentage









































































Comments
Post a Comment